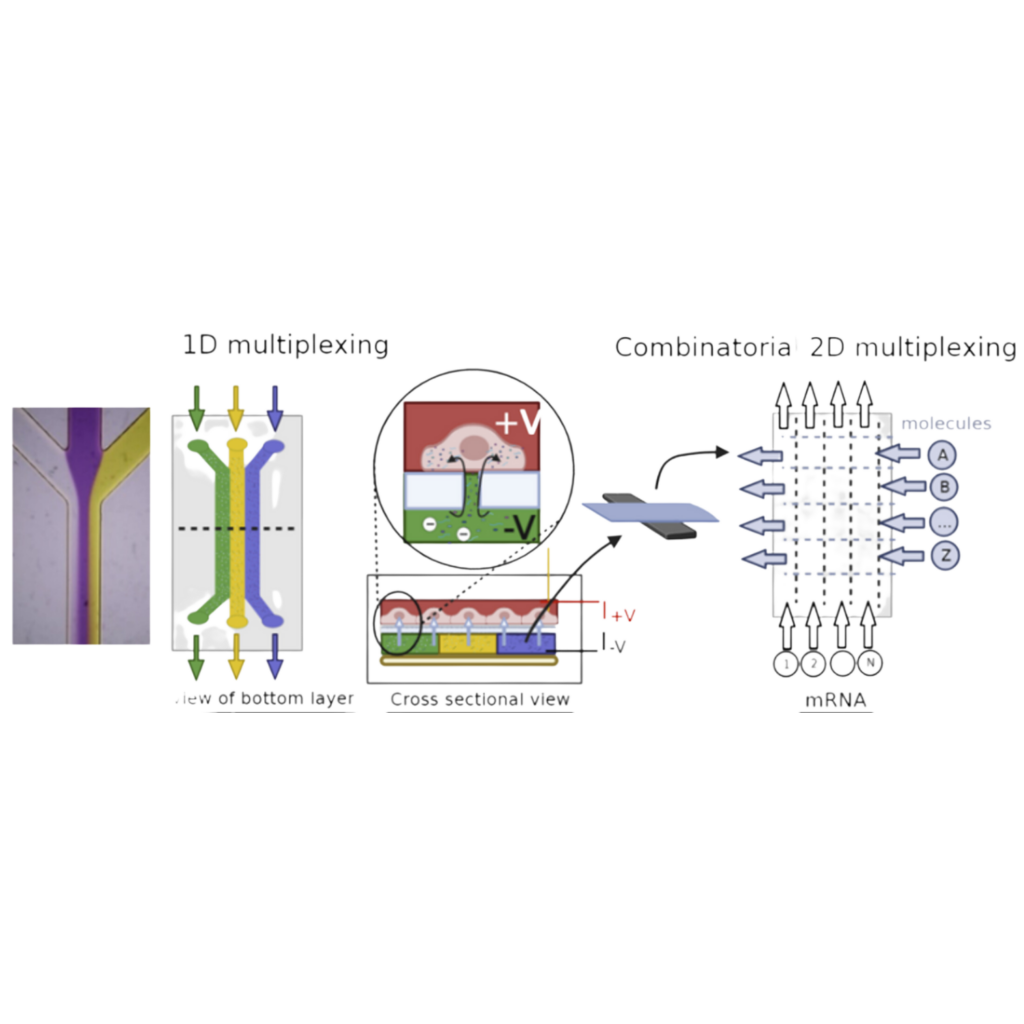
The efficient introduction of macromolecules, such as DNA and proteins, directly into cells provides a method to manipulate cells and is the crux of multiple biological tools and medical related applications, such as genetic engineering and therapies, cell imaging, and biophysical models. One effective method of introducing macromolecules is through electroporation, in which cell membranes are disrupted through an externally applied voltage, pores are generated across the cell, where macromolecules can diffuse into the cell before they close. However, traditional electroporation can be detrimental to cell health and function because of the non-specific disruption of the cell membrane in all directions. Recently, the use of a vertically aligned nanoporous membrane was used to localize the electroporation of cells cultured on top of this membrane. Electroporation only occurs at the nanopore-cell junction directly above the membrane, where the externally applied electric field is concentrated and magnified, limiting the disruption of the cell membrane and preserving cell viability (PNAS ref). <Add Figure>. Meanwhile, the macromolecular cargo can be accelerated in a charge-specific manner inside the vertically aligned nanopores, determined by the polarity of the external electric field, and be effectively “injected” into the cells. This bypasses the slow diffusion process in traditional electroporation which greatly enhances the delivery efficiency and specificity of desired macromolecular cargos. The use of these “electric-field-focusing” nanoporous membranes as site-specific nano-electroporators opens up the type of format cells can be electroporated in while also minimizing the negative effects of traditional electroporation. One exciting method we would like to develop is integrating the nano-electroporator method into microfluidic systems. By culturing cells atop these nanoporous membranes we aim to leverage the ability to flow different fluids under the cells, to produce a method of dynamic delivery of the macromolecules. However, as this platform has yet to be developed, there is little knowledge on how active flow can impact the nano-electroporation and consequential delivery of macromolecules into cells. We hypothesize that macromolecule delivery to cells is flow dependent and proportional to the velocity of the fluid, which can be used to control the quantity of delivery. Furthermore, due to the size scale of microfluidic systems, they have a unique feature of well-defined flow profiles, and the ability to “pattern” different liquids by using laminar flow streams. We hypothesize that by using flow rates to control the width of laminar flow streams, we can also dynamically tune the quantity and location of cells receiving different molecules in the microfluidic nano-electroporator. This work will combine microfluidic device design and fabrication, tissue culturing, electroporation, microscopy and image analysis to test our hypothesis. Our goal is to develop a tool that can enable dynamic cell modification and manipulation in order to systematically introduce differential macromolecules to tune the behavior of sub-populations of cells.
People

Albert
Liu
ChE
Engineering

Sasha Cai
Lesher-Pérez
BME, ChE
Engineering
Funding

Funding: $30K (2023)
Goal: We propose to develop Dynamic Nanopore Microfluidic Transfection via Electroporation (DyNaMiTE), that leverages microfluidics to introduce spatial and temporal control to nanopore electroporation as a therapeutic and diagnostic platform.
Token Investors: Albert Liu and Sasha Cai Lesher-Pérez
Project ID: 1106
